MonoSelect® Agnus: Clinically Studied Supplement to Empower Women Against PMS
Chasteberry extract, recognized by the EMA since 2010, is a potent ally against menstrual disorders and PMS, targeting prolactin and progesterone levels
A recent study examined MonoSelect® Agnus in managing PMS, showcasing significant symptom reduction, especially with continuous usage, offering a promising treatment avenue.
Chasteberry Extract: the Potential Solution for PMS featured in MonoSelect® Agnus
Since 2010, the ethanolic extract of Vitex agnus-castus fruits, commonly known as chasteberry extract, has garnered recognition from the European Medicines Agency (EMA) as a “well-established use” for treating menstrual cycle disorders and premenstrual syndrome (PMS). This acknowledgment is supported by robust scientific evidence attributing the efficacy of chasteberry to its modulation of prolactin and progesterone levels in women with PMS.
Recognizing the immense potential of chasteberry, PharmExtracta meticulously formulated MonoSelect® Agnus, a supplement aimed at maximizing the benefits of chasteberry extract. By standardizing the extract content and ensuring an optimal dosage of 40 mg per tablet, containing 0.5% agnuside, MonoSelect® Agnus guarantees efficacy, as demonstrated in a clinical trial. Its innovative fast-release technology facilitates rapid dissolution and absorption within 5 minutes, thereby enhancing its speed of action. Furthermore, MonoSelect® Agnus is a non-hormonal, gluten-free, and lactose-free solution, prioritizing safety and ease of use, while also boasting excellent tolerability and compliance
Randomized and Controlled Clinical Study on MonoSelect® Agnus
An Italian study titled Fast dissolving Agnus castus fruit extract for the premenstrual syndrome: A controlled clinical trial evaluated the efficacy of an ethanolic extract of chasteberry fruits within a rapid-release tablet, developed to optimize the extract’s action and marketed as MonoSelect Agnus®.
In this study, 82 women diagnosed with premenstrual syndrome, aged over 18 years, were randomized into two groups. The intervention group received MonoSelect® Agnus tablets once daily for 90 days, while the control group received 300 mg of magnesium oxide daily for the same period. The study continued for an additional 90 days, assessing symptom variation following treatment cessation or MonoSelect® Agnus intake only in the 7 days preceding menstruation.
The primary aim was to evaluate MonoSelect® Agnus’ efficacy in improving all PMS symptoms compared to magnesium oxide, using the Visual Analog Scale (VAS) based on the Scott-Huskisson model. The secondary aim was to assess the validity of a subsequent treatment regimen, involving MonoSelect® Agnus administration only in the 7 days preceding menstruation for another 3 months, by comparing symptoms with those of women who discontinued treatment.
MonoSelect® Agnus Attenuates All PMS Symptoms
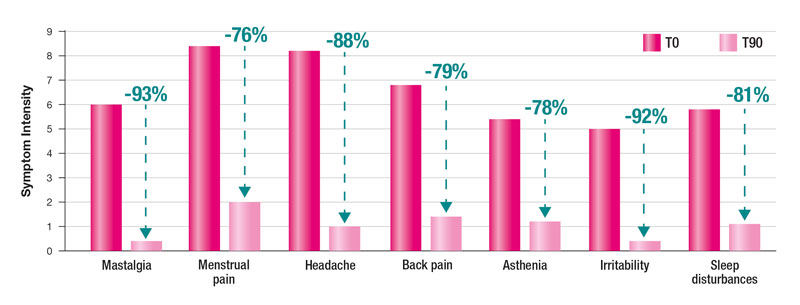
After the 90-day treatment period, women taking MonoSelect® Agnus exhibited significant improvements in all PMS symptoms compared to those taking magnesium oxide, which showed minimal efficacy. Symptom reduction percentages demonstrate MonoSelect® Agnus’ superiority in alleviating PMS symptoms.
Following complete cessation of MonoSelect® Agnus intake, symptoms reverted to baseline values after approximately one month of washout. Conversely, continuous intake of MonoSelect® Agnus in the 7 days preceding menstruation for another 3 months sustained benefits across all symptoms.
These results underscore the efficacy of MonoSelect® Agnus in managing all PMS symptoms effectively.